STANFORD RESEARCHERS ISOLATE SKELETAL STEM CELLS THAT GIVE RISE TO BONES AND CARTILAGE
Cartilage and bone deterioration are a common consequence of aging, but poor diet, sedentary lifestyle, excess weight or injury can also result in damaged tissue. Unlike bone tissue, mature cartilage is avascular and doesn’t heal well after injury. Replacement or augmentation surgery is one way to fix a torn joint, but the costs are high and there are also several risks involved in the procedure, such as transplant rejection and infection [1].
In January 2015, scientists at the Stanford University School of Medicine published a paper regarding their latest findings in tissue engineering. With the use of skeletal stem cells (myoblasts), they have been able to give rise to bone and cartilage in mice. In addition, they mapped out the chemical signals which can create skeletal muscle stem cells, directing their development into specialized types of cells [2].
To better understand the medical significance of these findings, we are going to take a closer look at stem cells and their role in bone and cartilage regeneration.
HOW SKELETAL STEM CELLS ARE OBTAINED
Stem cells (or blank cells) are undifferentiated cells that can divide or differentiate into specialized cells, replacing dying cells or damaged tissues. There are two broad types of stem cells: embryonic stem cells (ESCs) and adult stem cells (somatic stem cells).
ESCs are harvested from embryos 4-5 days post-fertilization, at each time they consist of 50-150 cells. Embryonic stem cells are pluripotent and can repair damaged tissue or stimulate the regeneration of diseased cells. However, due to ethical controversy, the study of ESCs is a slow process.
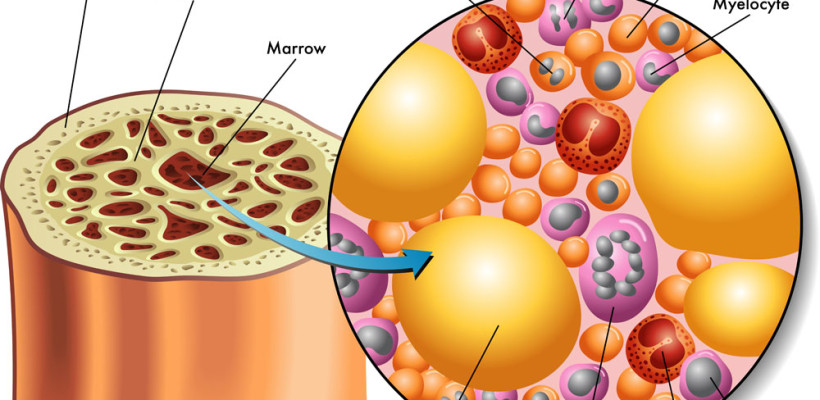
In humans, bone marrow, peripheral blood and adipose tissue are rich sources of adult stem cells, but these can be also harvested from some brain areas, skin, liver and even teeth. Until recent years, it was thought that adult stem cells differentiate only as the type of tissue they originate from. Emerging studies suggest that just like ESCs, these cells can specialize in unrelated cell types, as well.
The study conducted at the Stanford University School of Medicine supports these claims. The research focused on groups of cells with a fast division rate, located at the ends of mouse bones. Human skeletal muscle-derived cells were transplanted into host mice.
Prior to the procedure, the targeted host tissues were modulated by irradiation and cryoinjury, to allow the observation of the transplanted cells in mice. After four weeks of observation it was discovered that these isolated collections of cells were able to reconstruct all parts of the mouse bone.
Through further investigation, scientists were able to map the developmental tree of skeletal stem cells, which provided great insight on how to give rise to more specific types of cells. Irving Weissman, MD professor of pathology and of developmental biology, who directs the Stanford Institute for Stem Cell Biology and Regenerative Medicine, hopes that once these findings are translated into humans, the odds of rescuing cartilage and bone from wear and aging will increase significantly [3].
DIFFERENTIATION OF SKELETAL STEM CELLS AND THERAPEUTIC APPLICATIONS
Skeletal muscle is a dynamic tissue, capable of a regenerative response within a couple of weeks. This ability is primarily due to its satellite cells populations, a type of cells that are located peripheral to the myofiber.
When injury or disruption occurs, these satellite cells become activated and either fuse together to replace the damaged myofiber or multiply at an increased rate, supporting additional rounds of regeneration. In addition, skeletal stem cells can also give rise to blood derivatives, vascular components, osteoblasts (bone formation cells), adipocytes (fat cells) and cartilage [4].
The use of skeletal stem cells for therapeutic purposes brings hope to patients who suffer from muscular conditions, including muscular dystrophy. Joint pain, dislocations and arthritis are also on the list of potential stem cell therapy. Rheumatoid arthritis, Osteoarthritis and even Multiple Sclerosis patients could also benefit from these findings in the not-too-distant future.
The main challenge of using myoblasts for cell therapy remains, for now, harvesting and culturing them up to the numbers required.
References
[1] David King – Development and remodeling of skeletal tissue, School of Medicine, Southern Illinois University, 2009 http://www.siumed.edu/~dking2/ssb/skeleton.htm#development
[2] Christopher Vaughan – Researchers isolate stem cell that gives rise to bones, cartilage in mice, Stanford Institute for Stem Cell Biology and Regenerative Medicine, 2015 https://med.stanford.edu/news/all-news/2015/01/researchers-isolate-stem-cell-that-gives-rise-to-bones-cartilage.html
[3] http://www.stemcellclinic.net/tag/stanford
[4] http://genesdev.cshlp.org/content/20/13/1692.long




 Stem cells offer great potential for use in clinical applications thanks to their ability to specialize into different cell types and to renew themselves. Although some of them have limitations, stem cells are still an amazing resource for the medical world, as no other cell inside the human body has the ability to generate new cell types with a more specific function that the source.
Stem cells offer great potential for use in clinical applications thanks to their ability to specialize into different cell types and to renew themselves. Although some of them have limitations, stem cells are still an amazing resource for the medical world, as no other cell inside the human body has the ability to generate new cell types with a more specific function that the source.