
A New Medical Device, in the Management of Complex Wounds
Because of the complex nature of wound healing process, an injury on the skin can pose several challenges and are likely pose complications especially when they are acute. They can as well deteriorate from acute to chronic conditions which will require external intervention best understood by a specialist physician to get the area affected by the wound under normalcy.
The complexity of wound healing and research remains an ocean of knowledge that is continuously researched intensely to uncover depths of wound healing techniques and interventions. Hence, this report contains an introduction and details to the use of a new medical innovation called Gcells used primarily for the management of wounds in their different etiology.
In a case where the process of wound healing seemed difficult, Gcells proved great effects an attribute to their design and working protocol. Gcells are conditioned to work with an enriched suspension of progenitor cells that can efficiently aid tissue repair process. In this case report, two subjects were used as donors and acceptors of these micro-grafts.
Introduction
The skin is an outer layer of the body, offering protection to the underlying layers. A wound breaks this layer and inhibits the various functions as well as expose or also break the underlying layer of tissues. Repair processes are inherent and part of homeostatic processes of the body to try to restore the skin back to its normalcy in structure and in function.
The basics for the skins repair mechanism is represented by a cloth and an inflammation where vessels dilate and monocytes activate leading to breakdown of necrotic tissues. This basic process can be inhibited or delayed by a number of varying factors that lead to deteriorative transformation of acute wounds to chronic forms. But if there is no alteration in the repair process, Mesenchymal cells kickstart proliferative process and begin to repair and restructure the affected tissues starting from the base. At the same time epithelial tissues begin to grow around the wound leading to a final step of the healing process. In this final stage, remodeling of the skin structure is primary and then maturation of a scar.
These processes are efficient best in certain conditions which if affected by factors such as cardiovascular ailment, diabetes, bacterial or any other genre of infection, can inhibit these processes.
Hence, it is necessary to understand in details these processes if there is going to be development or innovation for effective healing processes. Just as stated above, during the proliferative phase of wound healing, Mesenchymal cells are the key role players. Their structure includes a Mesenchymal stem cell (MSCs), multi potent in nature and offer supportive, therapeutic and trophic functions. They are also able to release viable trophic, anti-inflammatory cytokines and anti-apoptotic molecules that offer protection during the repair of wounded skin. MSCs also possess subpopulations that are stem-like nature commonly referred to as “side population” (SP) they have been found out to be enriched in over 1000-fold of progenitor cells and multipotent stem cells and as well exist in tissues and tumors. SP exists in a variety of organs and tissues, after an original discovery to be prominent in the bone marrow of a mouse. The organs with SP include the lung, liver, brain, mammary gland and in skeletal muscles.
In other discoveries, it was discovered that they probably may also be isolated in other tissues of the body. This discovery was in an in vitro and in vivo experiment when Dental Pulp Stem Cells (DPSCs) showed capability to differentiate into osteoblasts and built a woven bone by forming an Extracellular Matrix (ECM) secreted by the osteoblasts. The experiment drew results on the both the quality and quantity of the matrix formed by the DPSCs in the in vivo and in vitro experiment using Stem cells and accompanying biomaterials.
Thus proved that dental pulp holds potentialities of therapeutic strength and a rich source of progenitor/autologous cells that can be used to aid healing processes even applicable to regeneration of craniofacial bones.
This is the evidence that supports the working principle of Gcells innovation. Gcell successfully separates this side population with a size of 50 micron. At this cell population, they can form autologous micro-grafts and can either be used alone or alongside biomaterials prepared in a biocomplex ready for use when necessary.
In this case report, two subjects were used as donors and acceptors of these micro-grafts for enhanced healing of complex wounds through autologous micro-grafts using the Gcell.
Clinical case 1
The first case involves a woman at age 50 who does not have any diseases or disorders. She underwent a laparoscopic gastric bypass surgery and was doing well considering parameters of weightloss. Two years later she moved in for abdominoplasty bariatric. Later on, post complications showed preeminence of necrosis which was discovered after first medical examination were about 150 to 200cm2 at the end of the flaps. An initial necrosectomy showed an intense loss of tissue and we furthered to place the wound on VAC therapy and the patient in active participation of this therapy for one week then at home as an outpatient.
As an outpatient, there was improved and progressive wound cleansing while granulated tissues around the base area were cleared.
The VAC therapy after 2 months still left the margins of the wound deteriorated and surrounding areas not in axis with skin surface.
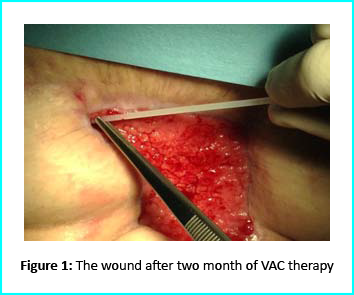
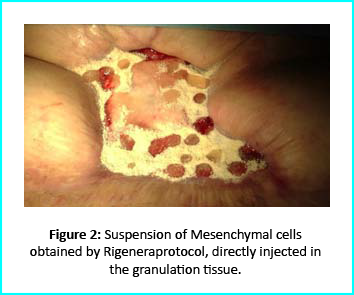
The Gcell protocol kickstarted after consent from the outpatient. We started by collecting a 3 cm2 skin sample from the patient for the purpose of obtaining the cell suspension needed to be injected to the granulation tissue (figure2).
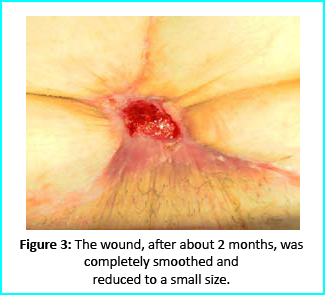
We followed up with conventional wound treatment as in cleaning and replacement with sterile gauze dabbed with Vaseline. The wound area began to improve in both healing progress and general appearance. In two months, the undermined area disappeared as well as leveled to the axis of the skin surface. 2 months later, the wound reduced to a very little scar that is mild and smoothed compared to the initial condition. (figure 3).
A man who suffered liver cirrhosis, hiatal hernia and diabetic as well at the age of 78. Complex surgery was carried out and distal esophagectomy was performed. But hiatal hernia was not decreased into the abdomen, so he was booked up for corrective surgery. During the intervention the adhesions correlated to the previous abdominal operation and led to opening the colon for resection. Some postoperative complications by the appearance of entero-cutaneous fistulas, related to a colonic anastomosis dehiscence. A second intervention was inevitable hence a ileostomy protection and repackaging of colonic anastomosis. We closed the laparotomy using a biological prosthesis. But we met further complications from ascetic failure that needed intensive care hepatology.
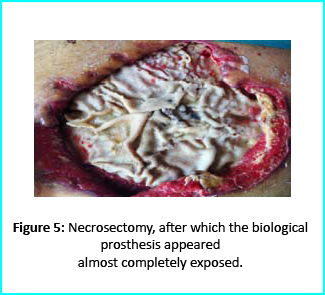
Patient’s condition that included poor liver synthesis had its toll on the healing of the surgical wound. Just as the first case, necrotic tissues grew around the biological prosthesis. We conducted necrosectomy and the biological prosthesis was left half exposed. (Figure 5).
Further treatment of the wound using advanced medication helped cover the biological prosthesis with granulation tissue (figure 6).
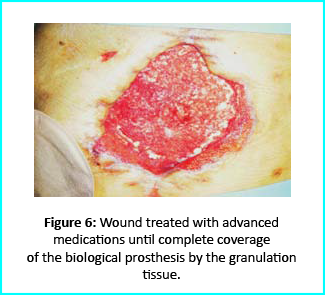
Plastic surgeons conducted evaluations on the patient and the choice to do a rotation flap did not seem so appropriate. VAC therapy was used on the wound for about 15 days even though the device wasn’t efficient enough to maintain supposed suction in the presence of ileostomy. We proceeded to treat the patient further with Gcell protocol when wound dimension progressed to about 250cm2. The tissue granulation was of right margin near the ileostomy improved even though it appeared to be undermined. In summary, Gcell protocol has proved a great level of efficiency in healing and restoration of damaged tissues. This progress is certain to open way for employment in the clinical practice that involves the treatment and management of acute and chronic wounds and in any other field of medicine that will inevitably need an instrument to repair lesion on tissues.
Discussion and conclusion
We made it clear earlier in this document about the efficiency of Gcell protocol in its aid to wound healing especially for wounds that are likely to develop from acute to chronic conditions. The working principle for the Gcell used to obtain the viable progenitor cells used for the micrografts relies on one individual as both the donor and the acceptor. This will help to reduce complications that are related to implants or injected micrografts that are non-autologous. Gcell is flexible and can be used both during in operating rooms as well as in ambulatories. This innovation is vastly spreading and currently used in the fields of oral-maxillo-facial field proven by recent studies even though a greater area of its application widespread and acceptable in plastic surgery, dermatology and orthopedics.
Conclusion of this report brings to clarity in demonstration, an efficient, useful and low-risk innovation in the field of medicine, useful for areas in wound management and healing. However, the viability of the Gcell product still needs to be texted on subjects with different conditions and perspectives. But we assure that this device will prove to be a better therapeutic approach in the field of medicine in improving healing of complex wounds. This confidence lies in the excellent features and working principles of this device in obtaining cell suspension, flexibility, facility for procedure and more importantly, the cost. This will help reduce the use of exorbitantly prices devices for advanced medication. In summary, apart from introducing an efficient innovation in the medicine. Gcell has the potentialities to offer employment on clinical procedures that will help aid in the management of wounds no matter how the case may be.

 s because it’s so important in learning, memory and mood function,” Shetty said. “We’re interested in understanding aging in the brain, especially in the hippocampus, which seems particularly vulnerable to age-related changes.”
s because it’s so important in learning, memory and mood function,” Shetty said. “We’re interested in understanding aging in the brain, especially in the hippocampus, which seems particularly vulnerable to age-related changes.” Shetty’s previous research focused on the benefits of resveratrol (an antioxidant that is famously found in red wine and the skin of red grapes, as well as in peanuts and some berries) to the hippocampus. Although the results indicated important benefits for preventing memory loss in aging brains, his newest work demonstrates a way to affect the function of the hippocampus more directly.
Shetty’s previous research focused on the benefits of resveratrol (an antioxidant that is famously found in red wine and the skin of red grapes, as well as in peanuts and some berries) to the hippocampus. Although the results indicated important benefits for preventing memory loss in aging brains, his newest work demonstrates a way to affect the function of the hippocampus more directly. In both old and young brains, a small percentage of the grafted cells retained their stemness feature—an essential characteristic of a stem cell that distinguishes it from ordinary cells—and continuously produced new neurons. This is called creating a new ‘niche’ of neural stem cells, and these niches seemed to be functioning well. They were still producing new neurons at least three months after implantation, and these neurons are capable of migrating to different parts of the brain.
In both old and young brains, a small percentage of the grafted cells retained their stemness feature—an essential characteristic of a stem cell that distinguishes it from ordinary cells—and continuously produced new neurons. This is called creating a new ‘niche’ of neural stem cells, and these niches seemed to be functioning well. They were still producing new neurons at least three months after implantation, and these neurons are capable of migrating to different parts of the brain.