![Secondary-Diabetes[1]](https://www.stemcellscourse.com/wp-content/uploads/2020/02/Secondary-Diabetes1.png)
Stem cell treatment could offer one-end-solution to Diabetes
Insulin-producing cells grown in the lab could provide a possible cure for the age-long disease (diabetes).
Type 1 diabetes is an auto¬immune disease that wipes out insulin-producing pancreatic beta cells from the body and raises blood glucose to dangerously high levels. These high levels of Blood sugar level can be even fatal. Patients are being administered insulin and given other medications to maintain blood sugar level. To those who cannot maintain their blood sugar level, they are given beta-cell transplants but to tolerate beta cell transplants; patients have to take immunosuppressive drugs as well.
A report by a research group at Harvard University tells us that they used insulin-producing cells derived from human embryonic stem cells (ESCs) and induced pluripotent stem cells to lower blood glucose levels in mice. Nowadays, many laboratories are getting rapid progress in human stem cell technology to develop those cells that are functionally equivalent to beta-cells and the other pancreatic cell types. Other groups are developing novel biomaterials to encapsulate such cells and protect them against the immune system without the need for immunosuppressant.
Major pharmaceutical companies and life sciences venture capital firms have invested more than $100 million in each of the three most prominent biotechnological industries to bring such treatments into clinical use:
- Cambridge
- Massachusetts–based companies Semma Therapeutics
- Sigilon Therapeutics, and ViaCyte of San Diego
Researchers of UC San Francisco have transformed human stem cells into mature insulin-producing cells for the first time, a breakthrough in the effort to develop a cure for type-1 (T1) Diabetes. Replacing these cells, which are lost in patients with T1 diabetes, has long been a dream of regenerative medicine, but until now scientists had not been able to find out how to produce cells in a lab dish that work as they do in healthy adults.
What is T1 diabetes?
T1 diabetes is an autoimmune disorder that destroys the insulin-producing beta cells of the pancreas, typically in childhood. Without insulin’s ability to regulate glucose levels in the blood, spikes in blood sugar can cause severe organ damage and eventually death. The condition can be managed by taking regular shots of insulin with meals. However, people with type 1 diabetes still often experience serious health consequences like kidney failure, heart disease and stroke. Patients facing life-threatening complications of their condition may be eligible for a pancreas transplant from a deceased donor, but these are rare, and they are supposed to wait a long time.
Researchers have just made a breakthrough that might one day make these technologies obsolete, by transforming human stem cells into functional insulin-producing cells (also known as beta cells) – at least in mice.
“We can now generate insulin-producing cells that look and act a lot like the pancreatic beta cells you and I have in our bodies,” explains one of the team, Matthias Hebrok from the University of California San Francisco (UCSF).
“This is a critical step towards our goal of creating cells that could be transplanted into patients with diabetes.”
Type-1 diabetes is characterized by a loss of insulin due to the immune system destroying cells in the pancreas – hence, type 1 diabetics need to introduce their insulin manually. Although this is a pretty good system, it’s not perfect.
Making insulin-producing cells from stem cells
Diabetes can be cured through an entire pancreas transplant or the transplantation of donor cells that produce insulin, but both of these options are limited because they rely on deceased donors. Scientists had already succeeded in turning stem cells into beta cells, but those cells remained stuck at an early stage in their maturity. That meant they weren’t responsive to blood glucose and weren’t able to secrete insulin in the right way.
Scientists at the University of California San Francisco made a breakthrough in the effort to cure diabetes mellitus type 1.
For the first time, researchers transformed human stem cells into mature insulin-producing cells, which could replace those lost in patients with the autoimmune. There is currently no known way to prevent type-1 (T1) diabetes, which destroys insulin production in the pancreas, limits glucose regulation, and results in high blood sugar levels. The condition can be managed with regular shots of insulin, but people with the disease often experience serious health complications like kidney failure, heart disease, and stroke.
“We can now generate insulin-producing cells that look and act a lot like the pancreatic beta cells you and I have in our bodies,” according to Matthias Hebrok, senior author of a study published last week in the journal Nature Cell Biology.
“This is a critical step toward our goal of creating cells that could be transplanted into patients with diabetes,” Hebrok, director of the UCSF Diabetes Center, said in a statement.
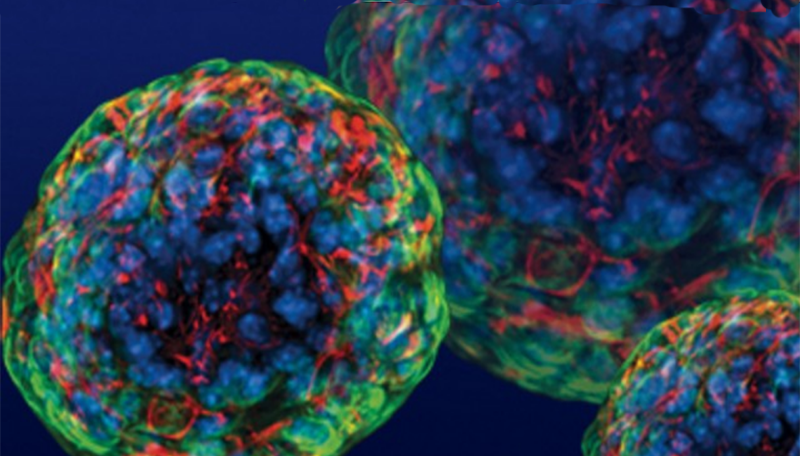
Islets of Langerhans are groupings of cells that contain healthy beta cells, among others. As beta cells develop, they have to separate physically from the pancreas to form these islets.
The team artificially separated the pancreatic stem cells and regrouped them into these islet clusters. When they did this, the cells matured rapidly and become responsive to blood sugar. In fact, the islet clusters developed in ways “never before seen” in a lab. After producing these mature cells, the team transplanted them into mice. Within days, the cells were producing insulin similar to the islets in the mice. While the study has been successful in mice, it still needs to go through more rigorous testing to see if it would work for humans as well. But the research is up-and-coming. “We can now generate insulin-producing cells that look and act a lot like the pancreatic beta cells you and I have in our bodies. This is a critical step towards our goal of creating cells that could be transplanted into patients with diabetes,” He said.
“We’re finally able to move forward on several different fronts that were previously closed to us,” he added. “The possibilities seem endless.”
Basic research keeps elucidating new aspects of beta cells; there seem to be several subtypes, so the gold standard for duplicating the cells is not entirely clear. Today, however, there is “a handful of groups in the world that can generate a cell that looks like a beta cell,” says Hebrok, who currently acts as scientific advisor to Semma and Sigilon, and has previously advised ViaCyte. “Certainly, companies have convinced themselves that what they have achieved is good enough to go into patients.”
The stem cell reprogramming methods that the three companies use to prompt cell differentiation create a mixture of islet cells. Beta cells sit in pancreatic islets of Langerhans alongside other types of endocrine cells. Alpha cells, for example, churn out glucagon, a hormone that stimulates the conversion of glycogen into glucose in the liver and raises blood sugar. Although the companies agree on the positive potential of islet cell mixtures, they take different approaches to developing and differentiating their cells. Semma, which was launched in 2014 to commercialize the Harvard group’s work and counts Novartis among its backers, describes its cells as fully mature, meaning that they are wholly differentiated into beta or other cells before transplantation. “Our cells are virtually indistinguishable from the ones you would isolate from donors,” says Semma chief executive officer BastianoSanna
To get around the donor problem, researchers, including the team at UCSF has been working on nudging stem cells into becoming fully-functional pancreatic beta cells for the last few years. Still, there have been some issues in getting them all the way there.
“The cells we and others were producing were getting stuck at an immature stage where they weren’t able to respond adequately to blood glucose and secrete insulin properly,” Hebrok said.
“It has been a major bottleneck for the field.”
“We’re finally able to move forward on a number of different fronts that were previously closed to us,” Hebrok added. “The possibilities seem endless.”
Regardless of starting cell type, the companies say they are ready to churn out their cells in large numbers. Semma, for example, can make more islet cells in a month than can be isolated from donors in a year in the United States, Sanna says, and the company’s “pristine” cells should perform better than donor islets, which are battered by the aggressive techniques required for their isolation.
As these products, some of which have already entered clinical trials, move toward commercialization, regulatory agencies such as the US Food and Drug Administration (FDA) and the European Medicines Agency have expressed concern about the plasticity of the reprogrammed cells. All three firms subject their cells to rigorous safety testing to ensure that they don’t turn tumorigenic. Before successful trials, companies won’t know the dose of beta cells required for a functional cure, or how long such “cures” will last before needing to be boosted. There’ll be commercial challenges, too: while the companies are investing heavily to develop suitable industrial processes, all acknowledge that no organization has yet manufactured cell therapies in commercial volumes.
Nevertheless, there’s growing confidence throughout the field that these problems will be solved, and soon. “We have the islet cells now,” says Alice Tomei, a biomedical engineer at the University of Miami who directs DRI’s Islet Immuno-engineering Laboratory.
“These stem cell companies are working hard to try to get FDA clearance on the cells.”
Protecting stem cell therapies from the immune system
Whatever the type of cell being used, another major challenge is delivering cells to the patient in a package that guards against immune attack while keeping cells fully functional. Companies are pursuing two main strategies:
- Microencapsulation, where cells are immobilized individually or as small clusters, in tiny blobs of a biocompatible gel.
- Macroencapsulation, in which greater numbers of cells are put into a much larger, implantable device.
ViaCyte, which recently partnered with Johnson & Johnson, launched its first clinical trial in 2014. The trial involved a micro-encapsulation approach that packaged up the company’s partially differentiated, ESC-derived cells into a flat device called the PEC-Encapsulation. About the size of a Band-Aid, the device is implanted under the skin, where the body forms blood vessels around it. “It has a semipermeable membrane that allows the free flow of oxygen, nutrients, and glucose,” says ViaCyte’s chief executive officer, Paul Laikind. “And even proteins like insulin and glucagon can move back and forth across that membrane, but cells cannot.”
The trial showed that the device was safe, well-tolerated, and protected from the adaptive immune system—and that some cells differentiated into working islet cells. But most cells didn’t engraft effectively because a “foreign body response,” a variant of wound healing, clogged the PEC-Encap’s membrane and prevented vascularization. ViaCyte stopped the trial and partnered with W. L. Gore & Associates, the maker of Gore-Tex, to engineer a new membrane. “With this new membrane,” says Laikind, “we’re not eliminating that foreign body response, but we’re overcoming it in such a way that allows vascularization to take place.” The company expects to resume the trial in the second half of this year, provided it receives the green light from the FDA.
Semma is also developing macro¬-encapsulation methods, including a very thin device that in prototype form is about the size of a silver dollar coin. The device is “deceptively simple, but it allows us to put [in] a fully curative dose of islets,” Sanna says.
Semma is also investigating microencapsulation alternatives. More info about all on 4 dental implants cost in Temecula you can find on www.temeculafacialoralsurgery.com/ site. At the same time, the company is advancing toward clinical trials using established transplantation techniques to administer donated cadaver cells to high-risk patients who find it particularly difficult to control their blood glucose levels. These cells are infused via the portal vein into the liver, and patients take immunosuppressive drugs to prevent rejection.
Sigilon is working on its microencapsulation technology. Launched in 2016 on the back of work by the labs of Robert Langer and Daniel Anderson at MIT, the company has created 1.5-millimeter gel-based spheres that can hold between 5,000 and 30,000 cells (Nat Med, 22:306–11, 2016). Each sphere is like a balloon, with the outside chemically modified to provide immune-protection, says Sigilon chief executive officer Rogerio Vivaldi. “The inside of the balloon is full of a gel that creates almost a kind of a matrix net where the cells reside.”
In 2018, shortly after partnering with Eli Lilly, Sigilon and collaborators published research showing that islet cells that were encapsulated in gel spheres and transplanted into macaques remained functional for four months. The company has not disclosed a time frame for a type 1 diabetes trial “but we’re moving pretty quickly,” says chief scientific officer David Moller.
Conclusion
To conclude, all three firms hope to extend their work to treat some of the 400 million people worldwide with type 2 diabetes, many of them eventually benefit from insulin injections. The recent endorsements from big Pharmaceutical underline the real progress in beta-cell transplants, says Aaron Kowalski, a molecular geneticist and chief executive officer at JDRF, a foundation based in New York that has funded research at ViaCyte and academic labs whose work has been tapped by Semma and Sigilon. “These companies all realize that if they don’t do it, somebody else will. It’s hard to predict exactly when, but somebody is going to make this work.”

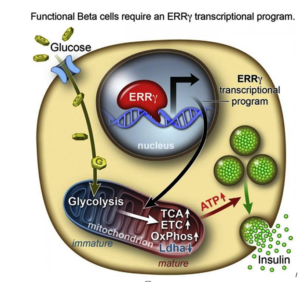
 After they genetically engineering a deficiency of ERRy in mice, the researchers found the animals’ beta cells did not produce insulin in response to spikes in blood sugar.
After they genetically engineering a deficiency of ERRy in mice, the researchers found the animals’ beta cells did not produce insulin in response to spikes in blood sugar.