Can Stem Cells treatment Regrow the knee cartilage?
Some people believe that stem cell does all of this but studies have shown that It’s unlikely only in a few unique cases. You can observe minimal growth a year after the patient took treatment, but this doesn’t mean replacement of the cartilage.
The cartilage has a reduced regenerative capacity, and current and present pharmacological medications only offer symptomatic pain relief. Osteoarthritis patients that respond poorly to conventional therapies are ultimately treated with surgical procedures to promote cartilage repair by implantation of artificial joint structures (arthroplasty) or total joint replacement (TJR). Surgery has been the last resort for serious cartilage problems.
In the last two decades, stem cells derived from various tissues with varying differentiation and tissue regeneration potential have been used for the treatment of osteoarthritis, damage to bones and others either alone or in combination with natural or synthetic scaffolds. The stem cells derived from these tissues primarily aid cartilage repair. Although stem cells can be differentiated into chondrocytes in vitro or aid cartilage regeneration in vivo, their potential for Osteoarthritis management remains limited as cartilage regenerated by stem cells fails to fully recapitulate the structural and biomechanical properties of the native tissue. It isn’t easy for the cartilage to regrow and assume its original biomechanical and structure form.
Apparently, Due to the limited intrinsic capacity of resident chondrocytes to regrow the lost cartilage post-injury, stem cell-based therapies have been proposed as a novel therapeutic approach for cartilage repair.
Also, stem cell-based therapies using mesenchyme stem cells (MSCs) or induced pluripotent stem cells (iPSCs) have been used successfully in clinical and preclinical situations.
Part of the issues associated with Mesenchyme stem cells can be averted by using iPSCs. iPSCs are an ideal patient-specific unlimited cell source for autologous tissue regeneration. With the Promising in vitro; studies have shown that vitro results have already been demonstrated in the cartilage engineering field for iPSCs. These were generated from various cell types.
What Is Cartilage and How Does It Get Damaged?
Cartilage is a connective tissue in the human body and body of other animals. In our joints, we have a few kinds of cartilage, but most often people refer to the smooth lining of a joint called articular or hyaline cartilage. This kind of cartilage gives rise to a soft layer of cushion on the end of a bone at the joint. The cushion is essential for balance, mechanical functions and athletics. This tissue of the cartilage is very strong, yet it can compress, readjust and absorb varying degrees of energy. It is also very slippery, smooth and flexible and these features allow the joint to glide effortlessly through a broad range of physical motions of any kind.
When joint cartilage is not working correctly or damaged, this smooth-cushioning-layer can be worn away, and this becomes a problem. In the case of traumatic injuries, sometimes a sudden force causes the cartilage to break off or poorly become damaged, exposing the underlying bone of the body. In the case of osteoarthritis (also called degenerative/wear-and-tear arthritis), over time that smooth layer can wear thin and uneven. Aging can also cause the cartilage to break off and certain life factors and diseases too, e.g. autoimmune diseases.
Eventually, as that cushion of the bones wears away, joint movements can become inflexible, stiff and painful on one or both legs (bones). Joints can even become inflamed and swollen. And as all these conditions, typically causes pain and limitations in activity become problematic. The action or activities that involve these bones leads to crushing pain and discomfort, depending on the severity of the case. Almost all activities involve the movement of bones; hence this condition is not an easy one.
There are some treatments for cartilage damage and arthritis. Although there some medicines, most of these treatments are focused either on relieving symptoms by smoothing down the damaged cartilage or concentrate on replacing the joint surface with an artificial implant. The later is for end-stage conditions, and the artificial plane is procedures such as knee replacement or hip replacement surgery.
How Can Stem Cells Help?
Stem cells are specialized cells that can multiply reform and develop into different types of tissue. In the developmental stages of a fetus, stem cells are plentiful and surplus. However, in adulthood, stem cells are restricted to specific tasks of regenerating a few types of cells, such as blood cells and liver cells in some cases of damage. There are almost no stem cells found in cartilage tissue, and therefore there is little to no capacity to heal or regrow new cartilage. For adults, the ability to regrow new cartilage is even more difficult due to age and lack of stem cells in the cartilages.
Most often, in the setting of orthopedic surgery and joint problems, stem cells are obtained from adult stem cell sources. The primary sources are bone marrow and fatty tissue. These stem cells can develop into cartilage cells, called chondrocytes.
They also exhibit some other helpful qualities by stimulating the body to reduce inflammation, stimulate cell repair, and improve blood flow. This process is caused by the secretion of cellular signals and growth factors to stimulate the body to initiate healing processes.
Once stem cells have been obtained, they need to be delivered to the area of the cartilage that damaged. One option is to inject the stem cells into the joint. There have been many studies investigating just this, and some data shows improvement in symptoms. How much of this improvement is the result of new cartilage growth versus other effects of stem cells (the healing properties listed above, including the anti-inflammatory effects) is unknown.
There is a challenge with giving stem cell injection. The problem with just injecting stem cells is that cartilage is a complex tissue that is comprised of more than only cells hence this can pose a challenge because the stem can’t regenerate all the things in the cartilage.
To regrow the cartilage, the complex tissue structure and biomechanics of cartilage must also be reconstructed to its former status. Cartilage can often /described as having a scaffold-like structure that is composed of water, cells, collagen, and proteoglycans, and infection-fighting antibodies. Injecting just the stem cells is thought to be less effective in stimulating the formation of the entire cartilage structure hence the challenge.
Some studies are investigating the types of 3-dimensional tissue scaffolds engineered to have a cartilage-like structure. The stem can then be injected into the scaffold, in hopes of better restoring a healthy type of cartilage. Three-dimensional printing is becoming an exciting part of this type of research. If everything works out as expected, the cartilage reconstruction could be achieved to a very high percentage.
How do stem cells work?
Necessarily, stem cells are progenitor cells which are capable of regeneration and differentiation into a wide range of specialized cell types. Once injected, stem cells follow inflammatory signals from damaged tissues and have multiple ways of repairing these damaged areas. It works as though the part is developing new; like what is seen during a child’s development.
The mesenchyme stem cells (MSCs) we are using are considered to be multipotent (they can transform into different cell types but cannot form an organ) but not pluripotent. In the body, these cells Do NOT function by transforming into different cell types or tissues.
They act via anti-inflammatory activity, immune modulating capacity, and the ability to stimulate regeneration. We go through a very high thorough screening process to find cells that we know have the best anti-inflammatory activity, the best immune modulating capacity, and the best ability to stimulate regeneration process on the tissue with damage.
ISSCA (International Society for Stem Cells Applications) www.issca.us
This is a business located in Miami, FL, where people around the world come to take a certification in the newest Stem Cells Protocols.
Some organizations have put in efforts to help discover some solutions in stem medicine. International Society for Stem Cell Application (ISSCA ) is one of the leading associations in setting standards and promoting excellence in the field of Regenerative Medicine, researches, publications related education, certification, research and publications.
The ISSCA is a unique-multidisciplinary community of physicians, stem specialist and scientists with a mission to advance the science, technology and practice of Regenerative Medicine. Their aim is to treat disease and lessen human suffering. ISSCA generally advances the specialty of Regenerative Medicine and serves its members.
The ISSCA provides certifications and standards in the practice of Regenerative Medicine as a medical specialty.
Although the expectation on this stem cell course is yet to be achieved; however, this is a part of medicine that can offer one-end-solution to various bone and body problems.
With the recent high-tech studies, efforts and dynamics, stem cell treatment can be a breakthrough in the future as its perspectives are very promising and unique. It is also not dangerous on the long-run.

 Duncan cautioned that transplantation of iPSC-derived liver cells is not yet ready for translation to the clinic, but the technology for sorting homogeneous liver cells can be used now to successfully and accurately model and study disease in the cell culture dish.
Duncan cautioned that transplantation of iPSC-derived liver cells is not yet ready for translation to the clinic, but the technology for sorting homogeneous liver cells can be used now to successfully and accurately model and study disease in the cell culture dish.

 The trials are designed to investigate the possible beneficial effect of freshly harvested bone marrow stem cell applications on knee osteoarthritis patients in the control group. Patients will receive standard treatment of bone marrow stem cells intravenously, and will be monitored and assessed for any changes in clinical condition.
The trials are designed to investigate the possible beneficial effect of freshly harvested bone marrow stem cell applications on knee osteoarthritis patients in the control group. Patients will receive standard treatment of bone marrow stem cells intravenously, and will be monitored and assessed for any changes in clinical condition.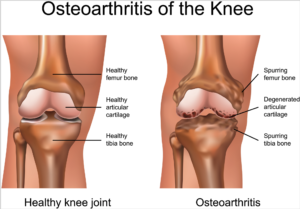 According to the
According to the