International Federation of Adipose Therapeutics and Sciences (IFATS)
45 Lyme Road – Suite 304
Hanover, NH 03755 USA
Tel: 1-603-643-2325, Fax: 1-603-643-1444
September 26, 2016
Division of Dockets Management (HFA–305) Food and Drug Administration
5630 Fishers Lane, Rm. 1061
Rockville, MD 20852
Re: FDA-2014-D-1856 – Comments to 2014-2015 Draft Guidance regarding:
- Docket FDA-2014-D-1584: “Same Surgical Procedure Exception under 21 CFR 1271.15(b): Questions and Answers Regarding the Scope of the Exception; Draft Guidance for Industry”;
- Docket FDA-2014-D-1696: “Minimal Manipulation of Human Cells, Tissues, and Cellular and Tissue-Based Products; Draft Guidance for Industry and Food and Drug Administration Staff”;
- Docket FDA-2014-D-1856: “Human Cells, Tissues, and Cellular and Tissue-Based Products from Adipose Tissue: Regulatory Considerations; Draft Guidance for Industry”;
- Docket FDA-2015-D-3581: “Homologous Use of Human Cells, Tissues, and Cellular and Tissue- Based Products; Draft Guidance for Industry and FDA Staff.”
Dear Sirs and Madams:
The International Federation of Adipose Therapeutics and Sciences (IFATS) appreciates this opportunity to submit the following comments to supplement its earlier written comments and recent testimony at the September 12-13, 2016 Public Hearing on the 2014-2015 Draft HCT/P Guidances concerning: a) Minimal Manipulation; b) Same Surgical Procedure; c) Adipose Tissue; and d) Homologous Use.
IFATS is committed to the responsible advance of the science and translation of new adipose therapies, and it is determined to ensure patient safety. It was founded in 2003 by pioneering adipose stem cell biologists and clinician–scientists with the goal of advancing the science of adipose tissue biology and its clinical translation to therapeutic applications. Since that time, IFATS has remained at the forefront of regenerative medical applications involving adipose tissue and cells. Membership now spans 40 countries in North America, Europe, Africa, the Middle East, Asia, Australia, and Central and South America, and includes basic scientists, translational researchers, clinicians, and regulatory and biotech representatives. IFATS is formally aligned with, and its members serve on the editorial boards of the prestigious journals, Stem Cells and Stem Cells Translational Medicine. With the International Society for Cellular Therapy (ISCT), IFATS has provided the scientific community with a detailed description and definition of adipose derived cells (both stromal vascular fraction, or SVF, and adipose-derived stromal/stem cells, or ASCs) in the formal publication entitled Cytotherapy. Thus, IFATS possesses the necessary expertise to assist regulatory agencies in understanding adipose tissue, and regulating the safety and efficacy of adipose-related products and therapies.
Drawing on this expertise, IFATS has reviewed the 4 draft guidances with great care. It respectfully requests the FDA to reconsider and modify the 4 draft HCT/P guidances as follows:
Recommendation #1 – Cell-Based Risks: Interpret and evaluate an HCT/P’s homologous use and minimal manipulation based on its manufacturer’s intended use in the patient.
Recommendation #2 – Provider-Based Risks: Reduce provider-created risks by targeting provider behavior.
Recommendation #3: Recognize that adipose HCT/Ps have both structural and nonstructural functions, and regulate based on its manufacturer’s intended use in the patient.
Recommendation #4: Revise the evaluation of minimal manipulation and homologous use as they pertain to particular applications of adipose tissue.
◊ ◊ ◊ ◊ ◊ ◊ ◊
IFATS recognizes the FDA’s challenge in developing regulations that fulfill the agency’s dual and interrelated responsibilities of protecting patients while promoting innovation. IFATS further recognizes that although these are complementary rather than competing objectives, they are often difficult to pursue simultaneously. The FDA’s 3-tiered, risk-based §§ 361 – 351 framework balances these concerns by making the degree of regulatory oversight proportionate to the degree of an HCT/P therapy’s risk.
The concepts of homologous use and minimal manipulation are key determinants of whether an HCT/P will be classified as a § 361 product (which does not need premarket approval) or a § 351 drug, device and/or biological product (which requires formal premarket approval). The applicability of § 351’s “same surgical procedure” exception also turns on homologous use and minimal manipulation. For most manufacturer-clinicians, § 351 categorization raises insurmountable obstacles due to the time and expense of obtaining premarket approval. In such cases, § 351 classification effectively prohibits access to safe and effective HCT/P therapies, even when those therapies involve a patient’s own cells and/or can deliver superior results with reduced risks. At the same time, § 351 oversight is essential for therapies that pose greater risks due the HCT/P’s characteristics, mechanism(s) of action and circumstances of use.
A second type of risk involves rogue clinicians offering false promise in the form of unproven therapies performed with few safeguards and less training. Provider misconduct is not unique to HCT/P therapies; it pervades all areas of medical practice. Nevertheless, IFATS shares the FDA’s alarm over such practices in the context of HCT/Ps, and is equally determined to curtail them. Because a solution cannot solve a problem without identifying and attacking its root cause, effective regulation of HCT/P-related risks must recognize and respond to their multivariate causes. Put simply:
- Sections 351 and 361 appropriately attempt to regulate HCT/P therapies proportionate to the risks of unpredictable and/or unsafe cell behavior.
- However, the risks of untrained providers misusing HCT/P therapies are caused by providers misbehaving, not cells misbehaving.
Consequently, interpretive guidance that restricts the definition and application of HCT/P terminology can only go so far in restricting provider-based risks. In addition, restrictive, inaccurate or imprecise definitions and interpretations carry their own risks of restricting access to therapies and restricting a patient’s right to evaluate risk through the process of informed consent.
Therefore, IFATS recommends that the FDA adopt an overall two-part strategy that focuses on both categories of HCT/P risks, i.e., those relating to cell behavior and those that pertain to provider behavior.
Recommendation #1 – Cell-Based Risks:
Interpret and evaluate an HCT/P’s homologous use and minimal manipulation based on its manufacturer’s intended use in the patient. Interpretive guidance should predicate each definition on to the functions and/or characteristics of the specific composition (i.e., cell type(s) and/or matrix or other component(s)) that are involved in, and/or relevant to the manufacturer- clinician’s intended use in the patient.
Recommendation #2 – Provider-Based Risks:
To reduce provider-created risks, the FDA should target provider behavior by collaborating with IFATS and comparable organizations to draw on and supplement existing federal and state methods of certification, registration, and similar measures.
Adopting this two-part strategy can control risk more comprehensively – and therefore more effectively – in furtherance of the FDA’s dual and interrelated obligations of protecting patients and promoting the availability of HCT/P therapies. IFATS explains each recommendation as follows:
Recommendation #1 – Cell-Based Risks: Interpret and evaluate an HCT/P’s homologous use and minimal manipulation based on its manufacturer’s intended use in the patient.
The four draft guidances on homologous use, minimal manipulation, same surgical procedure and adipose tissue individually and collectively intend to “improve stakeholders’ understanding” of 21 CFR 1271 by clarifying the FDA’s interpretation of homologous use and minimal manipulation. As demonstrated by the initial round of public comments and the ensuing public hearing on September 12 and 13, 2016, the draft guidance documents have not clarified applicable regulations. They have instead compounded the difficulty of understanding and complying with them. The drafts’ introduction of new definitional inaccuracies has also amplified rather than reduce patient risk.
IFATS respectfully requests the agency to clarify the definitions and application of homologous use and minimal manipulation by interpreting each as referring to the characteristics of the specific cell type(s) and/or the matrix or other component(s) that are involved in, and/or relevant to the manufacturer’s intended use in the patient. Thus, the definition of homologous use with interpretive guidance would read as follows:
21 CFR 1271.3(c): Homologous use means the repair, reconstruction, replacement, or supplementation of a recipient’s cells or tissues with an HCT/P that performs the same basic function or functions in the recipient as in the donor.
Recommended GUIDANCE: As used in this section, “performs the same basic function or functions in the recipient as in the donor” shall be interpreted as referring to one or more of the function(s) of the specific composition of the therapeutic/product, reflecting the specific cell type(s) and/or the specific matrix or other component(s) in the donor tissue that are involved in, and/or relevant to the manufacturer’s intended use in the patient.
Similarly, the definition of minimal manipulation with interpretive guidance would read as follows:
21 CFR 1271.3(f) Minimal manipulation means:
- For structural tissue, processing that does not alter the original relevant characteristics of the tissue relating to the tissue’s utility for reconstruction, repair, or replacement;
- For cells or nonstructural tissues, processing that does not alter the relevant biological characteristics of cells or
Recommended GUIDANCE: As used in this section, “relevant” characteristics shall be interpreted to mean the characteristics of the specific cell type(s) and/or the specific matrix or other component(s) in the donor tissue that are involved in, and/or relevant to the manufacturer’s intended use in the patient.
Rationale: Incorporating and relying on the manufacturer’s intended use harmonizes the interpretation and definition of homologous use and minimal manipulation with statutory directives to predicate the regulation of drugs, devices and biologics on the manufacturer’s intended use.
Defining relevant characteristics in terms of “the characteristics of specific cell type(s) and/or the matrix or other component(s) in the donor tissue that are involved in, and/or relevant to the manufacturer’s intended use in the patient” promotes patient safety by insisting on a reasonable and scientifically supportable rationale for using an HCT/P for a particular mechanism of action. This clarification balances the FDA’s dual responsibilities of protecting patients from undue safety risks while promoting the ongoing availability and continued development of HCT/P therapies.
Example of Non-Homologous Use: Decellularized adipose matrix used to accomplish the manufacturer’s intended use of a particular metabolic or systemic effect in the patient (e.g., reducing insulin levels in a diabetic patient) is non-homologous because decellularized matrix is not relevant to metabolic or systemic activity.
◊ ◊ ◊ ◊ ◊ ◊ ◊
Recommendation #2 – Provider-Based Risks: To reduce provider-created risks, the FDA should target provider behavior by collaborating with IFATS and comparable organizations to draw on, and supplement existing federal and state methods of certification, registration, and similar measures.
For a risk-reduction strategy to succeed, it must target the root cause of the risk. Revising, retracting or replacing interpretations of regulatory terminology can target the risks of cells behaving in an unsafe ways, but can do little to prevent providers from behaving in unsafe ways. Because the risks of irresponsible providers offering unsafe treatments are not exclusive to HCT/P therapies, many federal and state mechanisms already exist for identifying, disciplining and prohibiting clinics and clinicians from endangering patients.
IFATS shares the FDA’s concern about provider-related risks in the HCT/P sector and shares its determination to end or minimize these risks. IFATS respectfully requests the FDA to collaborate with it and comparable organizations to identify and draw on existing federal and state methods for curtailing provider misconduct, and developing additional protections in the form of provider certification, registration, monitoring and similar measures. At present, the §§ 351-361 regulatory framework does not – and cannot – adequately respond to this form of risk. Collaboration among stakeholders and coordination with existing means of provider oversight offers the most effective and efficient strategy for protecting patients from provider-created risk.
Therefore, IFATS respectfully requests the FDA to meet with IFATS, the American Association of Blood Banks and other accreditation bodies for the purpose of working together to identify provider-focused safety objectives and measures that can be translated into formal accreditation requirements and interpretive guidance.
◊ ◊ ◊ ◊ ◊ ◊ ◊
Recommendation #3: Recognize that adipose HCT/Ps have both structural and nonstructural functions, and regulate based on its manufacturer’s intended use in the patient.
IFATS requests the FDA to expand its definition of adipose tissue from exclusively structural in function to include both structural and/or nonstructural functions, depending on the manufacturer’s intended use in the patient. This modification is critically necessary in order to:
- Reconcile the interpretive guidance on the definition and regulation of adipose with applicable statutory and regulatory requirements;
- Reflect and ensure biological accuracy; and most importantly,
- Regulate an HCT/P’s risks based on the manufacturer’s intended use and mechanisms of action in the patient.
More specifically:
a. Recognizing adipose tissue’s structural and/or nonstructural functions is required by applicable statutory and regulatory requirements.
Adipose HCT/Ps must be defined as having structural and/or nonstructural functions to align the draft guidance with statutory and regulatory recognition that cells and tissues may have more than one function. According to 42 USC § 321(g)(1), “[t]he term ‘drug’ means … (B) articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in man or other animals; and (C) articles (other than food) intended to affect the structure or any function of the body of man or other animals; and (D) articles intended for use as a component of any article specified in clause (A), (B), or (C). (emphasis added). Statutory directives to focus on intended use pervade FDA regulation, including the regulation of drugs, biologics, devices, cosmetics, pesticides and more. Applicable statutes and regulations explicitly and implicitly recognize that the human body is complex, and its tissues and cells are often versatile and multi-functional. For example, 21 CFR 1271.3(c)’s definition of homologous use correctly recognizes that an HCT/P may have more than one “basic function.” It never says or even suggests that an HCT/P can only have one function, or that the regulator has sole authority to define that function and thereby dictate a manufacturer’s intended use. And yet the draft guidances do just that by insisting that adipose HCT/Ps are solely structural.
To align interpretive guidance with the regulations and statutory provisions being interpreted, IFATS respectfully requests the FDA to avoid pre-determining specific functions and uses for specific HCT/Ps. Instead, it should base regulations and guidance on the HCT/P’s function(s) and characteristic(s) that are relevant to its intended use by the manufacturer.
b. Recognizing adipose tissue’s structural and/or nonstructural functions is necessary to correct factual inaccuracy.
Regulation 21 CFR 1271.10(a)(4) categorizes an HCT/P as “either” structural or nonstructural, depending on its function. A structural HCT/P “does not have a systemic effect and is not dependent upon the metabolic activity of living cells for its primary function.” A nonstructural HCT/P “has a systemic effect or is dependent upon the metabolic activity of living cells for its primary function.”
The draft adipose guidance expressly acknowledges that adipose tissue contains adipocytes, preadipocytes, fibroblasts, vascular endothelial cells, a variety of immune cells, and also stores energy in the form of lipids. Citing only Junqueira ’s Basic Histology: Text & Atlas , the draft guidance classifies adipose as a connective and therefore structural tissue. This result is internally inconsistent and factually inaccurate – and the FDA’s sole cited authority explains why.
Junqueira classifies connective tissue as: 1) connective tissue proper; 2) embryonic connective tissues; and 3) specialized connective tissues. The latter category defines specialized connective based on their principal specialized functions. Blood, reticular connective tissue, adipose tissue, bone and cartilage all qualify as specialized connective tissues with specialized, nonstructural functions. Junqueira ’s examples include the following:
- Blood is a specialized connective tissue; its principal function of transport is nonstructural.
- Reticular connective tissues include the liver, pancreas, bone marrow and lymph They are nonstructural tissues because their principal functions are metabolic, including endocrine.
- According to Junqueira – the FDA’s sole cited authority – adipose tissue is nonstructural specialized connective tissue; its primary function is metabolic with co-existing structural
Junqueira ’s categorization of adipose as primarily nonstructural reflects longstanding scientific consensus. In1893, Gustav Neuber described his use of fat grafting in the orbital region to heal the adherent scarring which was the sequela of osteomyelitis. As a result of its nonstructural healing functions, the fat graft transformed facial scarring to more normal appearing skin and subcutaneous tissues. [6] In 1912, Holländer described the successful use of fat injections to prevent the recurrence of scarring following breast surgery. [7] In 1926, Charles Conrad Miller developed a new system for injecting fat grafts, and described 36 cases of correcting cicatricial contraction on the face and neck, and reported “excellent results” for another 2 cases after using fat grafts to treat “very persistent parotid fistulas…which defied all other methods of treatment.” [8] These and similarly favorable outcomes resulted from fat’s transformational nonstructural repair of the tissues into which it was placed. [8]
The understanding of the diverse roles of adipose tissue has steadily expanded [9], due in large part to the discovery of the first widely accepted adipokine, leptin, in the mid-1990’s. [10] Adipose tissue secretes proteins with systemic actions on hematopoietic, reproductive, metabolic, and other cells and tissues demonstrates unequivocally that adipose meets the definition of a true “endocrine” organ.[11,12] A Google scholar search of all available online medical and research databases for “the primary function of Adipose Tissue” returns 538,000 journal articles. Although the search did not designate a specific function, the search results referred to adipose tissue almost exclusively as a nonstructural metabolic and endocrine organ with secretory properties. A search for an exact match of the phrase “primary function of adipose tissue” yielded the following: “It was long believed the primary function of adipose tissue was energy storage; in fact, stromal adipose is a complicated endocrine organ.” However, even energy storage is nonstructural.
The FDA’s draft guidance on minimal manipulation defines nonstructural tissues as “serv[ing] predominantly metabolic or other biochemical roles in the body such as hematopoietic, immune, and endocrine functions.” To illustrate, the draft guidance offers “cord blood, lymph nodes, pancreatic tissue” as examples of nonstructural tissue. These tissues are indeed nonstructural – but they are also specialized connective tissue, as explained in Junqueira. In addition adipose has “hematopoietic, immune, and endocrine functions,” as explained below. As demonstrated by Junqueira, adipose HCT/Ps clearly do more than “reconstruction, repair, or replacement that relate to its utility to cushion and support the other tissues in the subcutaneous layer (subcutaneum) and skin.” And the FDA’s own nonstructural examples prove that classifying connective tissue, including adipose tissue as solely structural is factually inaccurate and logically flawed.
Thus, IFATS strongly recommends that the draft guidances be revised to define and categorize adipose tissue has both structural and nonstructural functions.
In support, IFATS offers the following examples of adipose’s nonstructural, and combined nonstructural and structural functions.
Nonstructural Functions of Adipose HCT/Ps:
- Nonstructural Endocrine Functions – It is well recognized that adipose is an endocrine organ which, like other endocrine organs, performs a variety of nonstructural Adipose tissue secretes proteins with nonstructural, systemic actions on hematopoietic, reproductive, metabolic, and other cells and tissues. [11, 12]
Examples:
- Glucose and lipid metabolism control via adipokine secretion [13]
- Reproductive and endocrine control via adipokine secretion [14-16]
- Immunomodulatory and immunosuppressive systemic control via cytokine and protein factor secretion [17-22]
2. Nonstructural Paracrine Functions
- Angiogenic control via vasculogenic cytokine secretion [22-26]
- Hematopoietic control via cytokine secretion, both locally and systemically [27]
- Neurogenesis via secretion of cytokine factors [28-34]
3. Nonstructural Hematopietic Potential of adipose stem cells in adipose deposits
- Reservoir for hematopoietic and lymphoid progenitor cells similar to bone marrow [18, 35, 36]
- Thermogenesis (brown and beige fat)[37-41]
- Energy reservoir (white adipose depots) [42,43]
4. Nonstructural Promotion of Lactation
- Fat serves as an energy reservoir and nutrient supply for breast epithelial cells.
- As pregnancy progresses, the breast epithelium proliferates in a branching manner to occupy the majority of the adjacent adipose tissue and
- At parturition, the epithelial cells draw on the lipid reserves of adipocytes within immediate proximity and secrete these nutrients into the milk available to the newborn infant during
- As long as the mother continues to breast feed the infant, the epithelial cells remain viable and
- If suckling is discontinued for periods of 24 to 48 hours, the epithelial cells undergo rapid apoptosis, leaving pre-adipocytes and adipocytes as the predominant cell within the breast
- While the presence and organization of epithelial cells within the breast tissue provide it with a unique architecture, the mammary adipocytes themselves show remarkable similarity to adipocytes from elsewhere in the Thus, the mammary fat pad displays homology to other adipose tissue depots.[44]
5. Nonstructural Regenerative Functions
- Local and circulating multipotent progenitor cells can repair and regenerate damaged tissues such as repairing irradiated skin, alleviating fibrotic changes, improving mobility and vitality, and repairing structures such as hair follicles and [45-47]. Specific examples include:
- Modulation of scarring
- Treatingold burn scars [55-57]
- Release of adherent scarring/fasciotomies [58]
- Modulation of scarring in primary cleft lip repair [59]
- Multipotent progenitor cells may be recruited for repair and regeneration of ischemic damage induced by acute myocardial infarction. [48]
- Adipose mesenchymal stem cells as progenitor cells in a perivascular position contribute to vascular network formation and vascular structures.[49-52] As such, the adipose mesenchymal stem cells are located in a position and serve a role shared by mesenchymal stem cells located in nearly all body tissues [53]. Adipose MSCs located in a range of tissues can enhance vascularity and perfusion, and thus provide cells that are precisely homologous to those already present in the
- Adipose mesenchymal stem cells induce a monocyte/macrophage phenotype switch from M1 to M2 macrophages, contributing to improved infarct healing post-acute myocardial [54]
6. Nonstructural Functions in Bone Marrow – Bone marrow and blood products are exempt from regulation under § 351 and 361. For over 40 years, it has been clearly established that adipose is present in bone marrow where it serves a wide variety of nonstructural functions. The following physiologic processes have nothing to do with providing cushioning and support and therefore are not properly categorized as a structural use or function of adipose cells. [3]
- Pre-adipocytes as mesenchymal cells in bone marrow: Bone marrow contains a spectrum of mesenchymal cells, including pre-adipocytes that can perform the nonstructural function of differentiating into adipocytes, osteoblasts and chondrocytes depending on the organism’s current needs.
- Pre-adipocytes and adipocytes regulate lympho-hematopoiesis and enable the bone marrow microenvironment to regulate proliferation within blood cell lineages to favor erythropoiesis rather than Adipocytes also contain nonstructural metabolic precursors and energy for the purpose of lympho-hematopoiesis. This is a nonstructural function.
- Adipocytes are essential for synthesizing plasma membranes during blood cell development because they contain cholesterol esters, triglycerides and lipoproteins.
- Bone marrow and extramedullary adipocytes are critical for homeostatic control of temperature in the bone marrow microenvironment and throughout the body, and thus contribute to the overall energy metabolism of the organism.
- Bone marrow adipose tissue is an essential endocrine organ.[4] Bone marrow adipose tissue (MAT) increases during caloric restriction (CR), is responsible for increased adipokine secretion, and alters skeletal muscle adaptation to These and other observations identify MAT as an endocrine organ.
BOTH Nonstructural and Structural Functions: In the following examples, adipose’s structural and nonstructural functions combine for the patient’s benefit:
- Reversal of damage caused by therapeutic radiation [60-63]
- Structural (filling tissue defect) uses, and
- Nonstructural tissue repair and regenerative uses [63]
- Treatingacute thermal injury [64-65]
- Treating Pain Mitigating implant breast pain [66]
- Improving post-mastectomy pain [67-68]
- Improving lower back pain [70]
- Nerveor neuroma repair [71-72]
- Healing ulcers
- Treatingpressure sores [73]
- Treating chronic non-healing anal fissures and associated stenosis [74]
- Treating vocal fold paralysis [75-77]
- Treating velopharyngeal insufficiency [78]
- Treating scleroderma and systemic sclerosis [79]
- TreatingDupuytren’s disease of the hand [80, 81]
- Treating Raynaud’s phenomenon – After fat grafting, there is improved symptomatology with evidence suggestive of measurably increased perfusion [82]
- Improving tendon repair
- Adipose tissue assists in tenolysis for foot and hand tendon [83]
- Treating adherent tendons and joints in burn patients with fat graft [84]
- Preventing osseous reunion of skull defects [85]
- Improvingthe quality of skin [86]
c. Regulating an HCT/P’s risks based on the manufacturer’s intended use and mechanisms of action in the patient ensures meaningful evaluation and effective regulation of risk.
The §§ 351-361 framework conditions the degree of regulatory oversight on the degree of an HCT/P’s risk. The homologous use and minimal manipulation criteria are central to determining whether an HCT/P will be classified as a § 361 or § 351 product, and if the latter, whether § 351’s “same surgical procedure” exception will apply. In turn, the existence of homologous use and minimal manipulation depend on the HCT/P’s structural or nonstructural function. More specifically:
21 CFR 1271.3(c) defines homologous use as “the repair, reconstruction, replacement, or supplementation of a recipient’s cells or tissues with an HCT/P that performs the same basic function or functions in the recipient as in the donor.”
21 CFR 1271.3(f) evaluates minimal manipulation of structural tissue in terms of processing that does not alter “the original relevant characteristics of the tissue relating to” the tissue’s utility for reconstruction, repair, or replacement. For nonstructural tissues, it evaluates “the relevant biological characteristics of cells or tissues.”
Insisting that adipose be evaluated as exclusively structural precludes any evaluation of its nonstructural functions despite their presence and importance in the donor and intended use and therapeutic benefits for the recipient. Failing § 361’s homologous use and minimal manipulation criteria are virtually guaranteed. This effectively prohibits any nonstructural use, and precludes any meaningful evaluation of their risks.
As a result, it effectively prohibits patient access to safe nonstructural applications of adipose tissue and thereby undermines the FDA’s obligations to protect patients and promote innovation.
The “ same surgical procedure” exception to § 351 also becomes completely unavailable for nonstructural use of adipose because it similarly requires homologous use and minimal manipulation.
◊ ◊ ◊ ◊ ◊ ◊ ◊
Recommendation #4: Revise the evaluation of minimal manipulation and homologous use as they pertain to particular applications of adipose tissue, (as detailed below).
IFATS respectfully requests the FDA to reconsider three particular applications of adipose tissue with regard to homologous use and minimal manipulation, each of which is required for § 361 classification as well as § 351’s “same surgical procedure” exception. In specific, IFATS requests the FDA to change its prior examples of the absence of homologous use and/or minimal manipulation to recognize the following:
- Decellularizing adipose tissue for structural use is minimal manipulation.
- Structural use of fat in the breast constitutes homologous
- Stromal vascular fractionation (SVF) of adipose to obtain nonstructural adipose components for use as a nonstructural tissue constitutes minimal
Each is explained in order
a. Decellularizing adipose tissue for structural use is minimal manipulation.
The draft guidance currently states that decellularizing structural adipose tissue constitutes more than minimal manipulation because the process alters the tissue’s ability to perform structural functions. This is incorrect. Adipose tissue’s structural functions are performed by a dense and interconnected skeleton of reticular fiber and dense connective tissue. Its biomechanical properties include tensile strength and elasticity, both of which are central to the structural functions of padding and cushioning.
Nonstructural components such as adipocytes, pre-adipocytes, lipids, etc. do not contribute to adipose’s structural characteristics or functions. It is well recognized that decellularization leaves adipose’s structural components fully intact. It does not alter, disturb or weaken the remaining reticular fiber and dense connective tissue skeleton, or compromise its ability to perform structural functions. Multiple reports have demonstrated that decellularized adipose tissue retains structural properties and can be injected to impart padding and cushioning of soft tissues. [89-93]
The FDA already classifies decellularized dermis as minimally manipulated, thereby acknowledging that the process of decellularization does not alter structural characteristics or functions of the remaining structural matrix. Removing cells from dermis and removing cells from adipose employ comparable methods to achieve comparable results. Decellularizing adipose for structural use, like decellularizing dermis for structural use, does not alter structural characteristics.
For these reasons, IFATS respectfully requests the FDA to revise the draft guidance to recognize that decellularized adipose is minimally manipulated as required by § 361 and § 351’s “same surgical procedure exception.”
b. Structural use of fat in the breast constitutes homologous use.
Example B-3 of the draft adipose guidance states that application of adipose-based HCT/Ps to the breast is nonhomologous use because “[t]he basic function of breast tissue is to produce milk (lactation) after childbirth. Because this is not a basic function of adipose tissue, using HCT/Ps from adipose tissues for breast augmentation would generally be considered a non-homologous use.” This logic is flawed and must be corrected because it mischaracterizes the function of the breast, and mischaracterizes the function of adipose in breast surgery.
- For the purpose of determining homologous use, the basic function of the breast is a secondary sex organ. In terms of shape, form and appearance, the breast is vital to a woman’s bodily integrity and body image, psychological sense of self, and overall physical and emotional health and well-being.
- Lactation is not the sole or even primary function of the breast.
- Most women never lactate, but their breasts do function as secondary sex organs throughout their adolescence and
- When lactation does occur, it is episodic, time-limited, and accounts for a very small fraction of a woman’s lifespan.
- Even when healthy, post-menopausal women cannot Restoring lactation is thus completely irrelevant to restoring breast function.
- All men have breasts, thousands develop breast cancer each year, and many will need reconstructive surgery — even though men do not lactate.
- Federal law recognizes and protects the breast’s importance as a secondary organ.
- The Women’s Health and Cancer Rights Act, 29 USC 1185b(a), requires group health insurers to cover “all stages” of breast reconstruction following mastectomy or irradiation, including bilateral correction of asymmetrical appearance where one breast is otherwise unaffected.
- Restoring lactation is not a goal or even a remote concern of this In fact, lactation is never mentioned in the statute’s text, legislative history or associated regulations.
- The function of adipose tissue in breast surgery is structural and therefore
- Mastectomy removes more than the ability to lactate. It removes size, shape and form by removing the breast mound, which is predominantly adipose. Consequently, applying adipose tissue for the structural purpose of restoring form and shape is homologous use.
- By classifying adipose based tissues as non-homologous when applied to the breast, an entire class of Centers for Medicare & Medicaid Services (CMS) approved breast reconstruction procedures would be at risk for not complying with the same surgical procedure For example:
- Autologous free tissue flap transfer (“free flap” breast reconstruction) is performed by transferring complex musculocutaneous flaps containing adipose One of the most common methods of reconstruction, it qualifies as an HCT/P because it completely removes fat-containing tissue flaps from the body before implanting. [94-96] Fat grafting for breast reconstruction is another common clinical practice.
- According to the draft adipose guidance, these and other methods of breast reconstruction could no longer be used without formal premarket approval because they do not restore lactation and are therefore non-homologous. Focusing solely on restoration of lactation ignores the fact that the breast is largely composed of fat tissue and its size, shape and form can be reconstructed with fat
- This and other methods of breast reconstruction will no longer be available for clinical use under 361 or § 351’s same surgical procedure exception because they will not restore lactation.
- Removing these and other reconstructive methods from clinical application has nothing to do with risk. It is instead a perverse outcome of insisting that breast reconstruction be evaluated for its ability to restore the breast’s minor and episodic function of lactation despite fat’s ability to restore the breast’s size, shape and function as a secondary sex organ.
For these reasons, IFATS respectfully requests the FDA to revise the draft HCT/P guidance documents to recognize that as applied to the breast, adipose tissue is homologous use because it performs the structural functions of restoring, repairing or reforming size, form and shape.
c. When intended for a nonstructural use in the patient, stromal vascular fractio n (SVF)cells should be evaluated as nonstructural when determining minimal manipulated and homologous use.
The FDA’s draft adipose guidance expressly acknowledges that adipose tissue contains a variety of nonstructural components, including adipocytes, preadipocytes, fibroblasts, vascular endothelial cells, a variety of immune cells, and also stores energy in the form of lipids. These are nonstructural because the cells perform the same regenerative functions in vivo as they do in vitro and animal models. [97- 98] Nonstructural adipose HCT/Ps are readily available in the stromal vascular fraction (SVF). Stromal vascular fractionation of lipoaspirate (typically obtained through liposuction) can remove fat’s structural components, making nonstructural SVF cells available for nonstructural use in a patient. Just as removing nonstructural cells through decellularization does not alter the relevant structural characteristics or structural function of the remaining structural matrix, removing structural components does not alter the relevant nonstructural characteristics or nonstructural function of the remaining nonstructural SVF components.
This is minimal manipulation under 21 CFR 1271.3(f)(2) because extracting nonstructural cells or tissues from lipoaspirate “does not alter the relevant biological characteristics of cells or tissues.”
Also, this is homologous use under 21 CFR 1271.3(c) because it uses lipoaspirate’s nonstructural HCT/Ps for “repair, reconstruction, replacement, or supplementation of a recipient’s cells or tissues with an HCT/P that performs the same basic function or functions in the recipient as in the donor.”
Examples: Nonstructural adipose tissue for homologous use, with minimal and more than minimal manipulation à Reversal of radiation damage as intended nonstructural use
Homologous use with no manipulation: Using liposuction aspirate to perform fat grafting/adipose tissue therapy for the intended use or of reversing radiation damage in the breast – a nonstructural function – is homologous use. The structural side-effect of increasing volume may be a collateral benefit, but the intended use is still nonstructural tissue repair.
- Use is homologous because the HCT/P performs that same basic nonstructural function in both donor and
Homologous use with minimal manipulation: Using liposuction aspirate is indicated for the nonstructural function of reversing radiation damage in the neck without the volume gain of a fat graft. Separating nonstructural from structural components obtains nonstructural SVF cells for nonstructural use in the patient.
- Use is homologous because it is performing the intended nonstructural function of reversing radiation
- Manipulation is minimal because processing does not alter relevant nonstructural biological characteristics.
Homologous use with more than minimal manipulation: Using liposuction aspirate is indicated for the nonstructural function of reversing radiation damage in the intestines by catheter injection of nonstructural SVF. However, an adequate dose is difficult to obtain because the patient is cachectic (low body fat caused by caloric depletion from radiation enteritis). Culture expansion is considered as a means of increasing dose.
- Use is again homologous because SVF cells would perform the intended nonstructural function of reversing radiation
- Manipulation is more than minimal because culture expansion of cells to yield a therapeutic dose alters relevant biological characteristic. SVF cells in their natural state do not engage in linear growth to create a homogeneous monoculture. Even tumors do not produce homogeneous monocultures.
Examples: SVF for nonhomologous use >>>Bone (re)generation
SVF cells do not normally form bone in their native location. Delivering SVF cells to bone for the intended structural function of directly (re)generating new bone (via action of “stem cells”) might be considered. Processing might involve combining SVF cells with one or more additives (such as ex vivo culture media additives) for the intended structural function of (re)generating NEW bone (such as additives added to our culture medias ex vivo). For this scenario:
- Use is nonhomologous because the basic function in donor and patient will differ if nonstructural SVF cells are combined with one or more additives (such as ex vivo culture media additives) for the intended structural function of (re)generating NEW bone (such as additives added to our culture medias ex vivo).
- Manipulation is more than minimal because processing would alter the nonstructural SVF’s original relevant characteristics.
◊ ◊ ◊ ◊ ◊ ◊ ◊
The members of IFATS are grateful for the FDA’s willingness to re-open and extend the period for public comments and allow additional time for the September 2016 public hearing on the 2014-2015 draft HCT/P draft guidances. As a multidisciplinary scientific society composed of adipose stem cell biologists and clinician–scientists, IFATS would greatly appreciate the opportunity to work with the FDA in meeting the challenges of regulating HCT/P therapies. We respectfully request that representatives of the FDA, including the Director of CBER, meet with members of IFATS to discuss the issues addressed herein as well as others that pertain to the advancement and regulation of adipose-based therapies.
Respectfully submitted on behalf of IFATS,
Adam J. Katz, MD, FACS
Chair, IFATS Regulatory Affairs Committee & IFATS Co-Founder University of Florida College of Medicine
Professor
Director of Plastic Surgery Research,
Laboratory of BioInnovation and Translational Therapeutics Division of Plastic Surgery, Department of Surgery
IFATS BOARD OF DIRECTORS
Bruce Bunnell, PhD
Tulane University / United States
Louis Casteilla, PhD
University of Toulouse / France
Sydney Coleman, MD
New York & Pittsburgh Universities / United States
Julie Fradette, PhD
Lavalle University / Canada
William Futrell, MD
Founders’ Board University of Pittsburgh / United States
Marco Helder, PhD
VU University Medical Center Amsterdam / The Netherlands
Adam J. Katz, MD, FACS
Founders’ Board University of Florida / United States
Ramon Llull, MD, PhD –
Founders’ Board University of Barcelona / Spain
Kacey Marra, PhD
University of Pittsburgh / United States
Ricardo Rodriguez, MD –
President (2016) Private Practice / Johns Hopkins / United States
Peter Rubin, MD, FACS – Chair, Founders’ Board Chairman of the Board
University of Pittsburgh / United States
Stuart K. Williams, PhD
University of Louisville / United States
MEMBERS–AT-LARGE
Jeff Gimble, MD, PhD
Pennington Biomedical / United States
Keith March, MD, PhD
Indiana University / United States
REFERENCES
- Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641-648
- Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L, Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909-969
- Gimble The function of adipocytes in the bone marrow stroma. The New Biologist. 1990;2:304-312
- Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell metabolism. 2014;20:368-375
- Meunier P, Aaron J, Edouard C, VlGNON Osteoporosis and the replacement of cell populations of the marrow by adipose tissue: A quantitative study of 84 iliac bone biopsies. Clinical orthopaedics and related research. 1971;80:147-154
- N. Über die wiederanheilung vollstädig vom körper getrennter, die ganze fettschicht en- thaltender hautstucke. Zbl f Chir 1893;30:16-17
- Hollander E, Joseph Cosmetic surgery. Handbuch der Kosmetik. Leipzig, Germany: Veriag von Velt. 1912;688
- Miller Cannula implants and review of implantation technics in esthetic surgery: In two parts. Oak Press; 1926.
- Gimble JM Fat circadian biology. Journal of applied physiology. 2009;107:1629-1637
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds Identification and expression cloning of a leptin receptor, ob-r. Cell. 1995;83:1263-1271
- Salgado AJ, Gimble Secretome of mesenchymal stem/stromal cells in regenerative medicine.
Biochimie. 2013;95:2195
- Salgado AJ, Reis RL, Sousa N, Gimble Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2009
- Khan M, Joseph Adipose tissue and adipokines: The association with and application of adipokines in obesity. Scientifica. 2014;2014
- Vicennati V, Garelli S, Rinaldi E, Di Dalmazi G, Pagotto U, Pasquali Cross-talk between
adipose tissue and the hpa axis in obesity and overt hypercortisolemic states. Hormone molecular biology and clinical investigation. 2014;17:63-77
- Kargi AY, Iacobellis Adipose tissue and adrenal glands: Novel pathophysiological mechanisms and clinical applications. International journal of endocrinology. 2014;2014
- Maïmoun L, Georgopoulos NA, Sultan Endocrine disorders in adolescent and young female athletes: Impact on growth, menstrual cycles, and bone mass acquisition. The Journal of Clinical Endocrinology
& Metabolism. 2014;99:4037-4050
- McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP,
Storms RW. The immunogenicity of human adipose‐derived cells: Temporal changes in vitro. Stem cells. 2006;24:1246-1253
- McIntosh KR, Frazier T, Rowan BG, Gimble Evolution and future prospects of adipose- derived immunomodulatory cell therapeutics. Expert review of clinical immunology. 2013;9:175-184
- McIntosh KR, Lopez MJ, Borneman JN, Spencer ND, Anderson PA, Gimble Immunogenicity of allogeneic adipose-derived stem cells in a rat spinal fusion model. Tissue Engineering Part A. 2009;15:2677-2686
- Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G. Immunophenotype of human adipose‐derived cells: Temporal changes in stromal‐associated and stem cell–associated markers. Stem cells. 2006;24:376-385
- Gimble JM, Dorheim MA, Cheng Q, Medina K, Wang CS, Jones R, Koren E, Pietrangeli C, Kincade Adipogenesis in a murine bone marrow stromal cell line capable of supporting b lineage lymphocyte growth and proliferation: Biochemical and molecular characterization. European journal of immunology. 1990;20:379-387
- Frazier TP, McLachlan JB, Gimble JM, Tucker HA, Rowan Human adipose-derived stromal/stem cells induce functional cd4+ cd25+ foxp3+ cd127− regulatory t cells under low oxygen culture conditions. Stem cells and development. 2014;23:968-977
- Frazier TP, Gimble JM, Kheterpal I, Rowan Impact of low oxygen on the secretome of human adipose- derived stromal/stem cell primary cultures. Biochimie. 2013;95:2286-2296
- Miranville A, Heeschen C, Sengenes C, Curat C, Busse R, Bouloumie Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292-1298
- Planat-Benard V, Silvestre J-S, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez Plasticity of human adipose lineage cells toward endothelial cells physiological and therapeutic perspectives. Circulation. 2004;109:656-663
- Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, Halvorsen YD, Cheatham B, Storms RW, Gimble Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro- inflammatory factors. J Cell Physiol. 2007;212:702-709
- Ribeiro CA, Fraga JS, Grãos M, Neves NM, Reis RL, Gimble JM, Sousa N, Salgado The secretome of stem cells isolated from the adipose tissue and wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res Ther. 2012;3:18
- Silva NA, Gimble JM, Sousa N, Reis RL, Salgado Combining adult stem cells and olfactory ensheathing cells: The secretome effect. Stem cells and development. 2013;22:1232-1240
- Cho YJ, Song HS, Bhang S, Lee S, Kang BG, Lee JC, An J, Cha CI, Nam DH, Kim. Therapeutic effects of human adipose stem cell‐conditioned medium on stroke. Journal of neuroscience research. 2012;90:1794-1802
- Egashira Y, Sugitani S, Suzuki Y, Mishiro K, Tsuruma K, Shimazawa M, Yoshimura S, Iwama T, Hara The conditioned medium of murine and human adipose-derived stem cells exerts neuroprotective effects against experimental stroke model. Brain research. 2012;1461:87-95
- Wei X, Du Z, Zhao L, Feng D, Wei G, He Y, Tan J, Lee WH, Hampel H, Dodel Ifats collection:The conditioned media of adipose stromal cells protect against hypoxia‐ischemia‐induced brain damage in neonatal rats. Stem Cells. 2009;27:478-488
- Wei X, Zhao L, Zhong J, Gu H, Feng D, Johnstone B, March K, Farlow M, Du Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neuroscience letters. 2009;462:76-79
- Zhao L, Wei X, Ma Z, Feng D, Tu P, Johnstone B, March K, Du Adipose stromal cells- conditional medium protected glutamate-induced cgns neuronal death by bdnf. Neuroscience letters. 2009;452:238-240
- Cousin B, André M, Arnaud E, Pénicaud L, Casteilla Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochemical and biophysical research communications. 2003;301:1016-1022
- Han J, Koh YJ, Moon HR, Ryoo HG, Cho CH, Kim I, Koh Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood.2009
- Harms M, Seale Brown and beige fat: Development, function and therapeutic potential. Nature medicine. 2013;19:1252-1263
- Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology. 2013;154:2687-2701
- Wu J, Cohen P, Spiegelman Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes & development. 2013;27:234-250
- Krings A, Rahman S, Huang S, Lu Y, Czernik P, Lecka-Czernik Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546-552
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500-1508
- Peirce V, Carobbio S, Vidal-Puig The different shades of fat. Nature. 2014;510:76-83
- Enerbäck S, Gimble Lipoprotein lipase gene expression: Physiological regulators at the transcriptional and post-transcriptional level. Biochimica et Biophysica Acta (BBA)- Lipids and Lipid Metabolism. 1993;1169:107-125
- Rudolph MC, Neville MC, Anderson Lipid synthesis in lactation: Diet and the fatty acid switch. Journal of mammary gland biology and neoplasia. 2007;12:269-281
- Gimble JM, Katz AJ, Bunnell Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249-1260
- Bellows CF, Zhang Y, Chen J, Frazier ML, Kolonin Circulation of progenitor cells in obese and lean colorectal cancer patients. Cancer Epidemiology Biomarkers & Prevention. 2011;20:2461- 2468
- Bellows CF, Zhang Y, Simmons PJ, Khalsa AS, Kolonin Influence of bmi on level of circulating progenitor cells. Obesity. 2011;19:1722-1726
- Krijnen PA NB, Meinster E, Vo K, Musters RJ, Kamp O, Niessen HW,, Juffermans LJ. Acute myocardial infarction does not affect functional characteristics of adipose derived stem cells in rats, but reduces the number of stem cells in adipose tissue. IFATS Annual Meeting. 2014:100
- Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH,March KL. A population of multipotent cd34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circulation research. 2008;102:77-85
- Traktuev DO, Prater DN, Merfeld-Clauss S, Sanjeevaiah AR, Saadatzadeh MR, Murphy M, Johnstone BH, Ingram DA, March Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circulation research. 2009;104:1410-1420
- Merfeld-Clauss S, Gollahalli N, March KL, Traktuev Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Engineering Part A. 2010;16:2953-2966
- Merfeld-Clauss S, Lupov IP, Lu H, Feng D, Compton-Craig P, March KL, Traktuev. Adipose stromal cells differentiate along a smooth muscle lineage pathway upon endothelial cell contact via induction of activin a. Circulation research. 2014;115:800-809
- Crisan M, Yap S, Casteilla L, Chen C-W, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3:301-313
- Ter Horst E, Naaijkens B, Krijnen P, Van Der Laan A, Piek J, Niessen Induction of a monocyte/macrophage phenotype switch by mesenchymal stem cells might contribute to improved infarct healing postacute myocardial infarction. Minerva cardioangiologica. 2013;61:617-625
- Guisantes E, Fontdevila J, Rodríguez Autologous fat grafting for correction of unaesthetic scars. Annals of plastic surgery. 2012;69:550-554
- Klinger M, Caviggioli F, Klinger FM, Giannasi S, Bandi V, Banzatti B, Forcellini D, Maione L, Catania B, Vinci Autologous fat graft in scar treatment. Journal of Craniofacial Surgery. 2013;24:1610-1615
- Klinger M, Marazzi M, Vigo D, Torre Fat injection for cases of severe burn outcomes: A new perspective of scar remodeling and reduction. Aesthetic plastic surgery. 2008;32:465-469
- Khouri RK, Smit JM, Cardoso E, Pallua N, Lantieri L, Mathijssen IM, Khouri Jr RK, Rigotti Percutaneous aponeurotomy and lipofilling: A regenerative alternative to flap reconstruction? Plastic and reconstructive surgery. 2013;132:1280-1290
- Balkin DM, Samra S, Steinbacher Immediate fat grafting in primary cleft lip repair. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2014;67:1644-1650
- Rigotti G, Marchi A, Galie M, Baroni G, Benati D, Krampera M, Pasini A, Sbarbati. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plastic and reconstructive surgery. 2007;119:1409-1422
- Villani F, Caviggioli F, Klinger F, Klinger Rehabilitation of irradiated head and neck tissues by autologous fat transplantation. Plastic and reconstructive surgery. 2009;124:2190-2191
- Chang CC, Thanik VD, Lerman OZ, Saadeh PB, Warren SM, Coleman SR, Hazen Treatment of radiation skin damage with coleman fat grafting. STEM CELLS. 2007;25:3280-3281
- Sultan SM, Stern CS, Allen Jr RJ, Thanik VD, Chang CC, Nguyen PD, Canizares O, Szpalski C, Saadeh PB, Warren Human fat grafting alleviates radiation skin damage in a murine model. Plastic and reconstructive surgery. 2011;128:363-372
- Loder S, Peterson JR, Agarwal S, Eboda O, Brownley C, DeLaRosa S, Ranganathan K, Cederna P, Wang SC, Levi Wound healing after thermal injury is improved by fat and adipose-derived stem cell isografts. Journal of Burn Care & Research. 2015;36:70-76
- Sultan SM, Barr JS, Butala P, Davidson EH, Weinstein AL, Knobel D, Saadeh PB, Warren SM, Coleman SR, Hazen Fat grafting accelerates revascularisation and decreases fibrosis following thermal injury. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2012;65:219-227
- Cuomo R, Zerini I, Botteri G, Barberi L, Nisi G, D’ANIELLO Postsurgical pain related to breast implant: Reduction with lipofilling procedure. In Vivo. 2014;28:993-996
- Maione L, Vinci V, Caviggioli F, Klinger F, Banzatti B, Catania B, Lisa A, Klinger Autologous fat graft in postmastectomy pain syndrome following breast conservative surgery and radiotherapy. Aesthetic plastic surgery. 2014;38:528-532
- Caviggioli F, Maione L, Forcellini D, Klinger F, Klinger Autologous fat graft in postmastectomy pain syndrome. Plastic and reconstructive surgery. 2011;128:349-352
- Caviggioli F, Vinci V, Codolini Autologous fat grafting: An innovative solution for the treatment of post-mastectomy pain syndrome. Breast Cancer. 2013;20:281-282
- Salgarello M, Visconti The role of sacrolumbar fat grafting in the treatment of spinal fusion instrumentation-related chronic low back pain: A preliminary report. Spine. 2014;39:E360-E362
- Faroni A, Terenghi G, Reid Adipose-derived stem cells and nerve regeneration: Promises and pitfalls. Int Rev Neurobiol. 2013;108:121-136
- Vaienti L, Gazzola R, Villani F, Parodi Perineural fat grafting in the treatment of painful neuromas. Techniques in hand & upper extremity surgery. 2012;16:52-55
- Marangi GF, Pallara T, Cagli B, Schena E, Giurazza F, Faiella E, Zobel BB, Persichetti Treatment of early-stage pressure ulcers by using autologous adipose tissue grafts. Plastic Surgery International. 2014;2014
- Lolli P, Malleo G, Rigotti Treatment of chronic anal fissures and associated stenosis by autologous adipose tissue transplant: A pilot study. Diseases of the Colon & Rectum. 2010;53:460-466
- Cantarella G, Baracca G, Forti S, Gaffuri M, Mazzola Outcomes of structural fat grafting for paralytic and non-paralytic dysphonia. Acta Otorhinolaryngologica Italica. 2011;31:154
- DeFatta RA, DeFatta RJ, Sataloff Laryngeal lipotransfer: Review of a 14-year experience. Journal of Voice. 2013;27:512-515
- Sataloff Autologous fat implantation for vocal fold scar. Current opinion in otolaryngology & head and neck surgery. 2010;18:503-506
- Cantarella G, Mazzola RF, Mantovani M, Baracca G, Pignataro Treatment of velopharyngeal insufficiency by pharyngeal and velar fat injections. Otolaryngology– Head and Neck Surgery. 2011;145:401-403
- Papa N, Luca G, Sambataro D, Zaccara E, Maglione W, Gabrielli A, Fraticelli P, Moroncini G, Beretta L, Santaniello A. Regional implantation of autologous adipose tissue-derived cells induces a prompt healing of long-lasting indolent digital ulcers in patients with systemic sclerosis. Cell transplantation. 2014
- Hovius SE, Kan HJ, Smit X, Selles RW, Cardoso E, Khouri Extensive percutaneous aponeurotomy and lipografting: A new treatment for dupuytren disease. Plastic and reconstructive surgery. 2011;128:221-228
- Verhoekx JS, Mudera V, Walbeehm ET, Hovius Adipose-derived stem cells inhibit the contractile myofibroblast in dupuytren’s disease. Plastic and reconstructive surgery. 2013;132:1139-1148
- Bank J, Fuller SM, Henry GI, Zachary Fat grafting to the hand in patients with raynaud phenomenon: A novel therapeutic modality. Plastic and reconstructive surgery. 2014;133:1109-1118
- Damgaard OE, Siemssen Lipografted tenolysis. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2010;63:e637-e638
- Colonna M, Scarcella M, d’Alcontres F, Delia G, Lupo Should fat graft be recommended in tendon scar treatment? Considerations on three cases (two feet and a severe burned hand). European review for medical and pharmacological sciences. 2014;18:753-759
- Merikanto JE, Alhopuro S, Ritsilä Free fat transplant prevents osseous reunion of skull defects: A new approach in the treatment of craniosynostosis. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery. 1987;21:183-188
- Mojallal A, Lequeux C, Shipkov C, Breton P, Foyatier J-L, Braye F, Damour Improvement of skin quality after fat grafting: Clinical observation and an animal study. Plastic and reconstructive surgery. 2009;124:765-774
- Lockwood Superficial fascial system (sfs) of the trunk and extremities: A new concept. Plastic and reconstructive surgery. 1991;87:1009-1018
- Song AY, Askari M, Azemi E, Alber S, Hurwitz DJ, Marra KG, Shestak KC, Debski R, Rubin Biomechanical properties of the superficial fascial system. Aesthetic Surgery Journal. 2006;26:395-403
- Flynn The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715-4724
- Brown BN, Freund JM, Han L, Rubin JP, Reing JE, Jeffries EM, Wolf MT, Tottey S, Barnes CA, Ratner Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Engineering Part C: Methods. 2011;17:411-421
- Wu I, Nahas Z, Kimmerling KA, Rosson GD, Elisseeff An injectable adipose matrix for soft tissue reconstruction. Plastic and reconstructive surgery. 2012;129:1247
- Omidi E, Fuetterer L, Mousavi SR, Armstrong RC, Flynn LE, Samani Characterization and assessment of hyperelastic and elastic properties of decellularized human adipose tissues. Journal of biomechanics. 2014;47:3657-3663
- Wang L, Johnson JA, Zhang Q, Beahm Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering. Acta biomaterialia. 2013;9:8921-8931
- Healy C, Allen Sr The evolution of perforator flap breast reconstruction: Twenty years after the first diep flap. Journal of reconstructive microsurgery. 2014;30:121-125
- LoTempio MM, Allen Breast reconstruction with sgap and igap flaps. Plastic and reconstructive surgery. 2010;126:393-401
- Erić M, Mihić N, Krivokuća Breast reconstruction following mastectomy; patient’s satisfaction. Acta Chir Belg. 2009;109:159-166
- Diaz-Flores L, Gutierrez R, Lizartza K, et Behavior of In Situ Human Native Adipose Tissue CD34+ Stromal/Progenitor Cells During Different Stages of Repair. Tissue-Resident CD34+ Stromal Cells as a Source of Myofibroblasts. Anatomical record. 2014.
- Gil-Ortega M, Garidou L, Barreau C, et Native adipose stromal cells egress from adipose tissue in vivo: evidence during lymph node activation. Stem cells. 2013;31(7):1309-20.

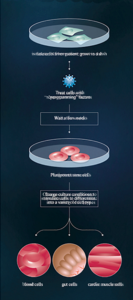
 Clearly, no one is going to convert embryonic stem cells into neurons and reprogram them into induced stem cells. Such a process would be too time-consuming and expensive. This experiment simulated the reprogramming of a patient’s
Clearly, no one is going to convert embryonic stem cells into neurons and reprogram them into induced stem cells. Such a process would be too time-consuming and expensive. This experiment simulated the reprogramming of a patient’s 

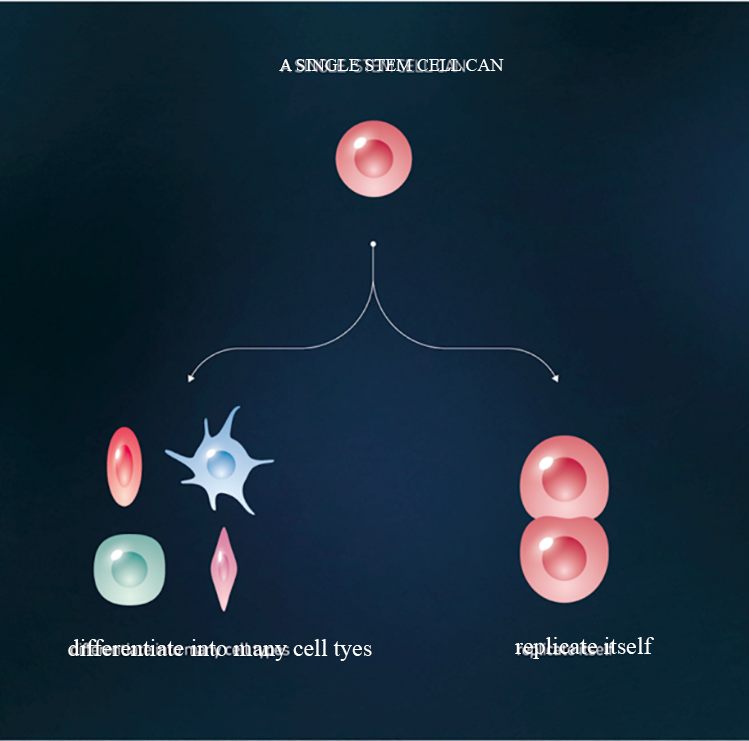
 Adult stem cells (non-embryonic) are found throughout the body that multiply by cell division to replenish dying cells and regenerate damaged tissues.
Adult stem cells (non-embryonic) are found throughout the body that multiply by cell division to replenish dying cells and regenerate damaged tissues.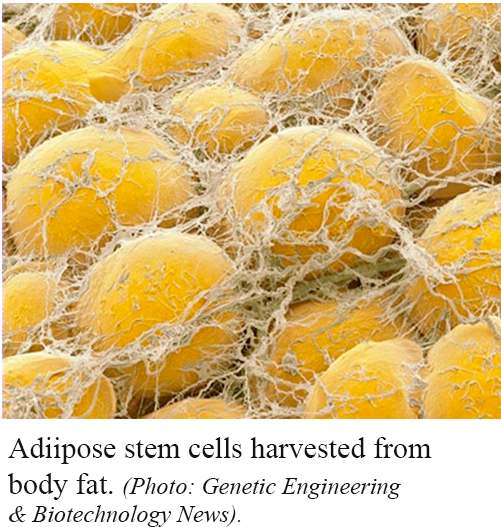
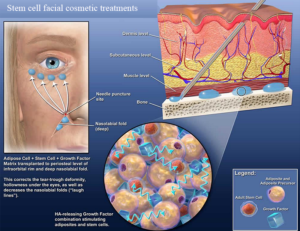
 Over the past 10 years, plastic surgeons have established safe and convenient ways to remove fat and isolate the stem cells for use in cosmetic procedures. And since adipose stem cells are extracted and reintroduced to the patient’s own body, the risk of rejection that goes with donor stem cells is eliminated. Scores of ongoing clinical trials using adipose stem cells have already proven their safety and efficacy in a variety of applications. Anti-aging therapies using adipose stem cells, for instance, have grown exponentially in popularity.
Over the past 10 years, plastic surgeons have established safe and convenient ways to remove fat and isolate the stem cells for use in cosmetic procedures. And since adipose stem cells are extracted and reintroduced to the patient’s own body, the risk of rejection that goes with donor stem cells is eliminated. Scores of ongoing clinical trials using adipose stem cells have already proven their safety and efficacy in a variety of applications. Anti-aging therapies using adipose stem cells, for instance, have grown exponentially in popularity.

 taking on a new challenge—unifying the powerful hospitals that train Harvard’s medical students.
taking on a new challenge—unifying the powerful hospitals that train Harvard’s medical students.

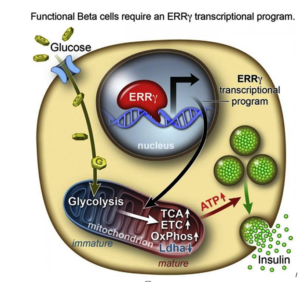
 After they genetically engineering a deficiency of ERRy in mice, the researchers found the animals’ beta cells did not produce insulin in response to spikes in blood sugar.
After they genetically engineering a deficiency of ERRy in mice, the researchers found the animals’ beta cells did not produce insulin in response to spikes in blood sugar.